Orthoss®
Orthoss® is a bone graft substitute that is bio-engineered to closely resemble the inorganic component of human bone tissue. It is an apatite similar to the structure of the mineral phase of human bone. Orthoss® is produced in Switzerland, following a rigorous quality assurance system to ensure its safety and quality.
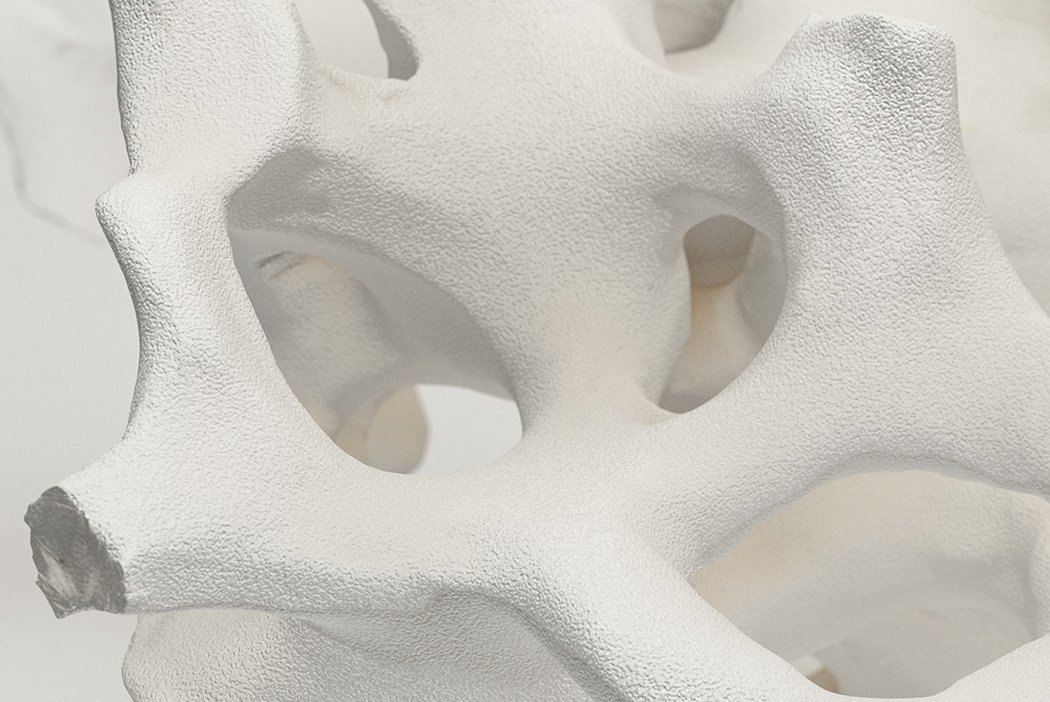
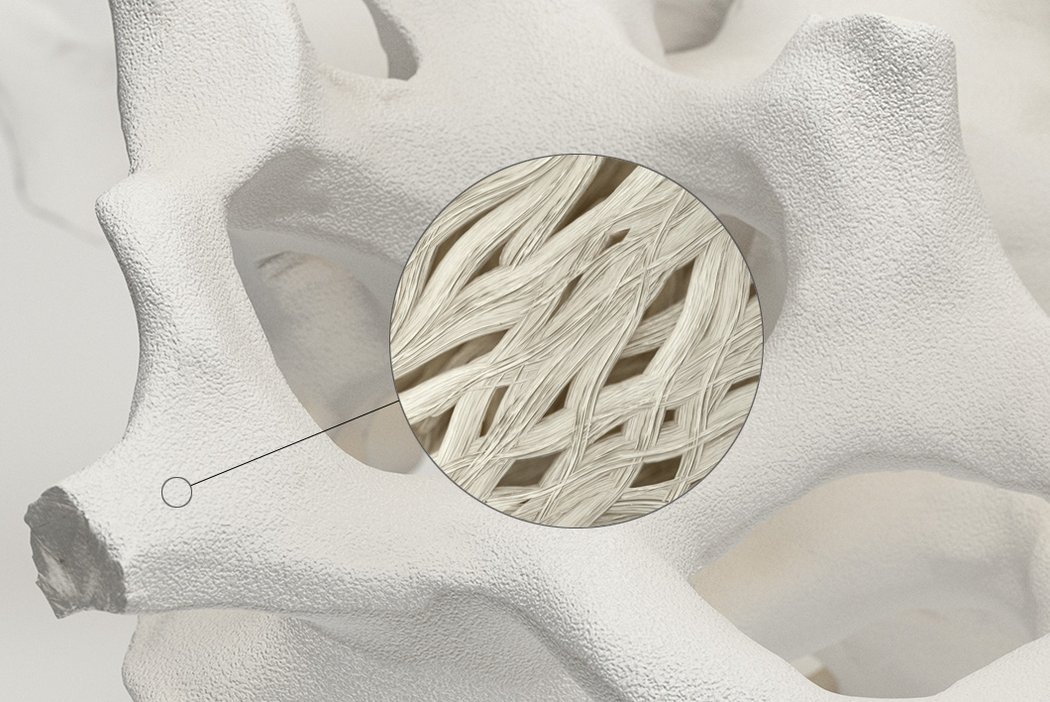
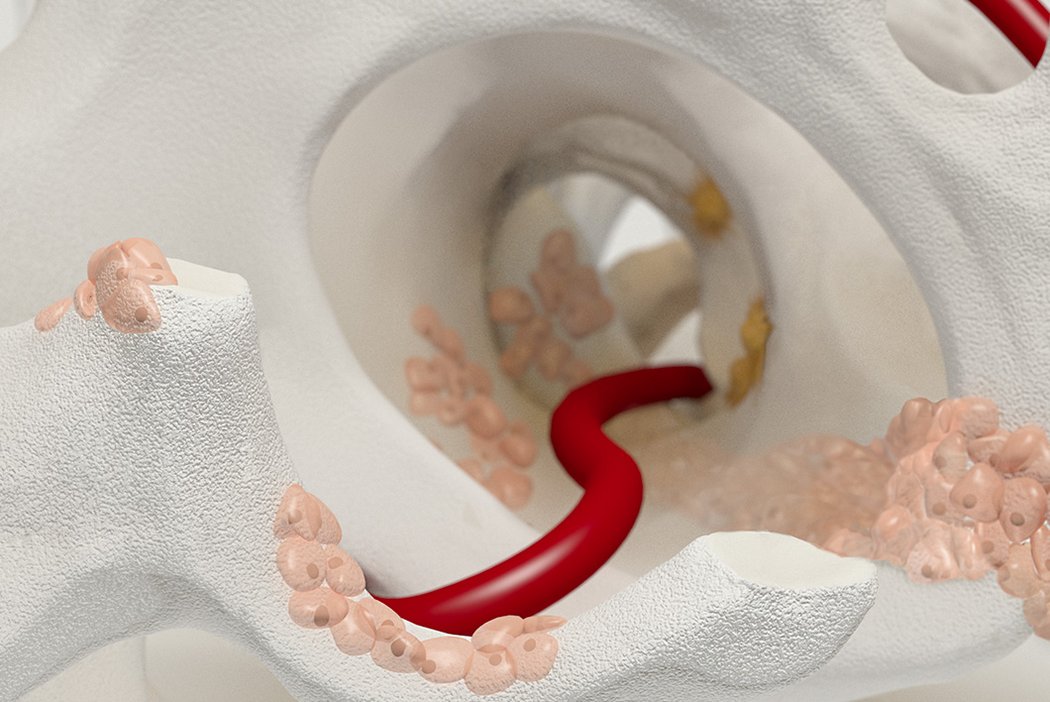
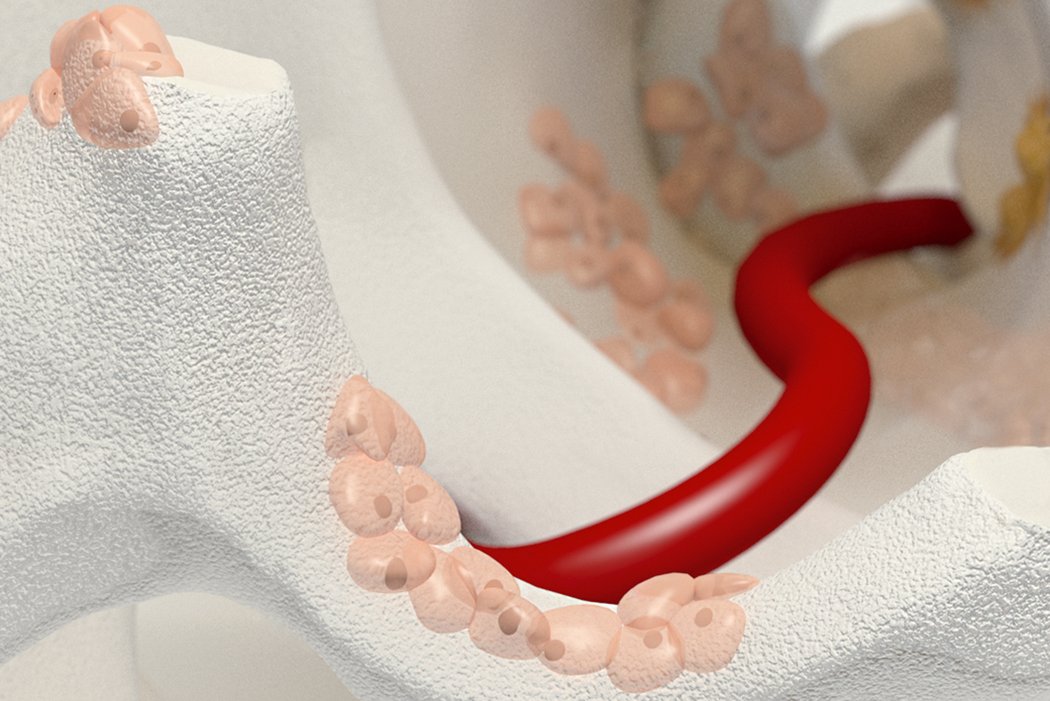
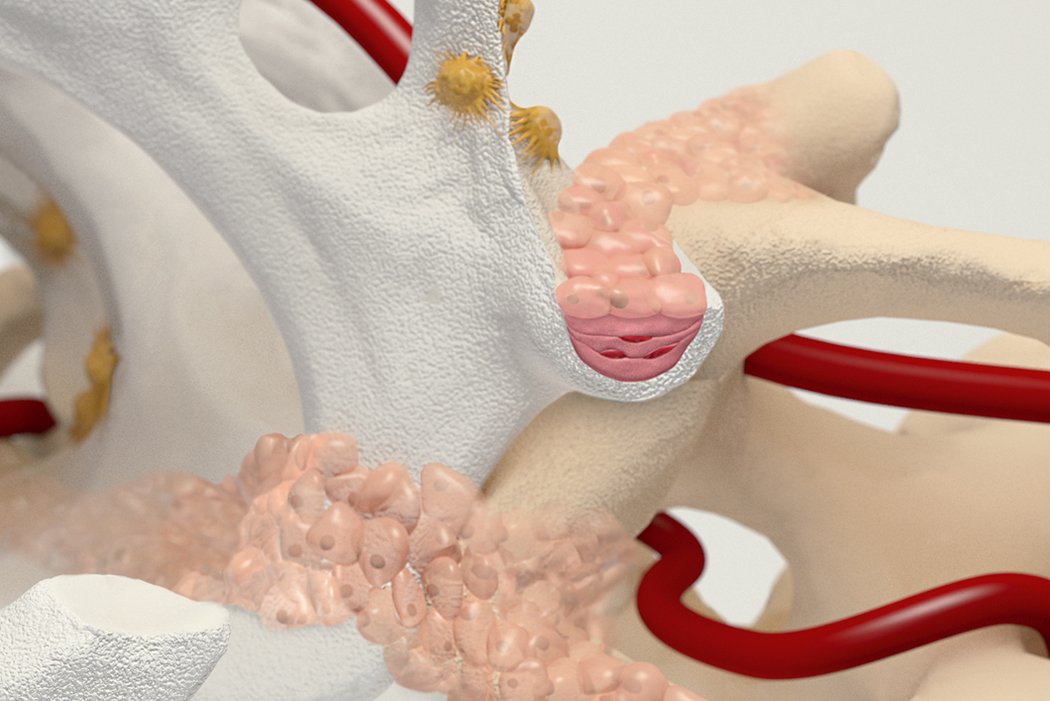
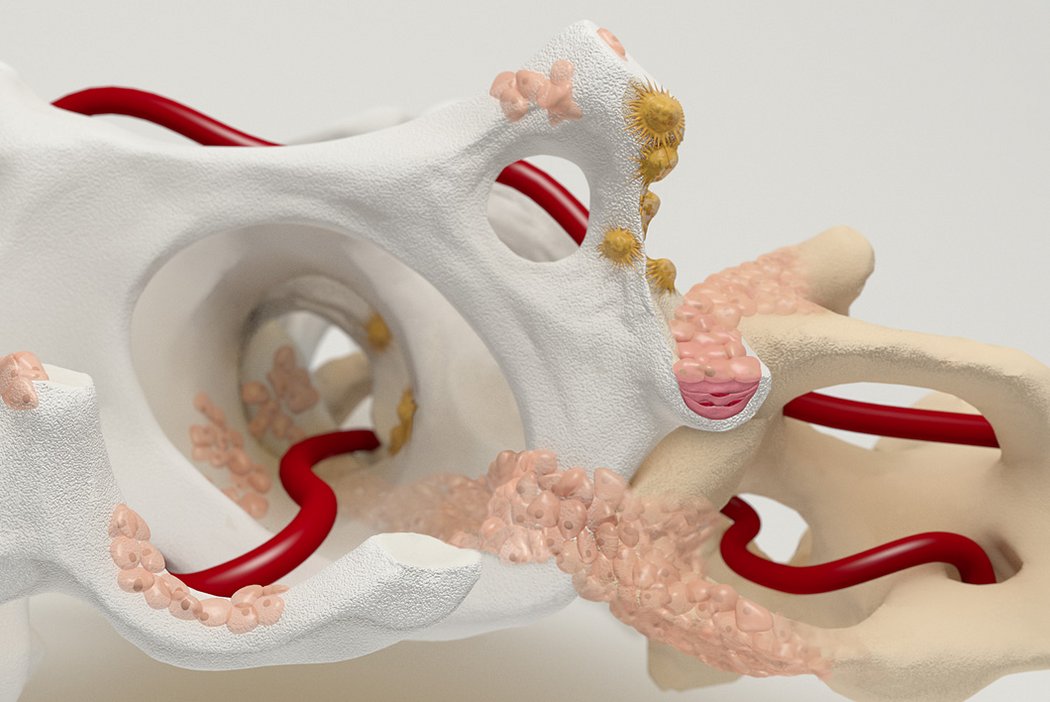
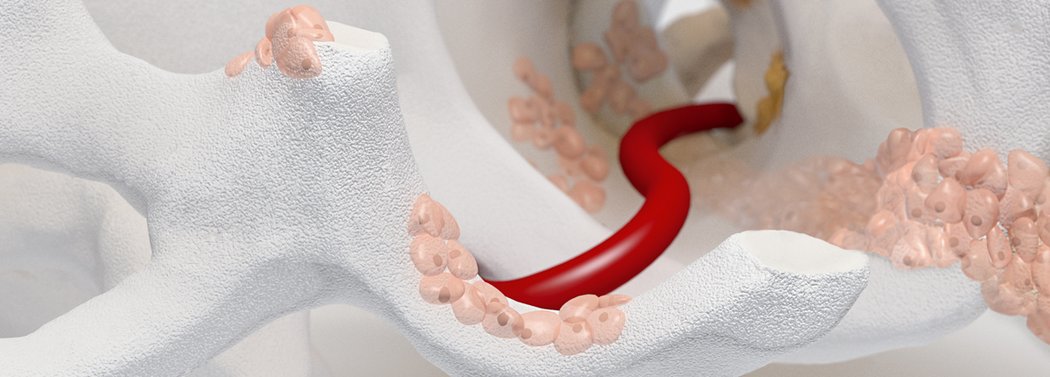
Orthoss® features a unique pore structure that consists of both micropores and macropores. Each acts as a conduit for the elementsbneeded for bone regrowth – including blood.
Backed by 25+ years of clinical experience, Orthoss® offers the osteoconductive properties of human bone in a convenient, off the shelf configuration. 1,2
The product was designed in collaboration with surgeons and has an extensive global history demonstrating the safety of this material in orthopedic and dental indications.
Orthoss® has been used in a variety of orthopedic applications and features a structure that supports bone regeneration.
References:
- Schlickewei, W. et al. Hefte zur Unfallkunde,216,(1991).(Case series)
- Bereiter, H. et al. (1991). Hefte zur Unfallkunde,216.(Expert opinion)
- Data on file at Geistlich Pharma AG, Wolhusen, Switzerland.(Bench Test)
- Kouroupis, D. et al. (2013). J Orthop Res. 31(12): 1950–8.(In Vitro)
- Orsini, G. et al. (2005). J Biomed Mater Res B Appl Biomater. 74(1): 448–7.(Case series)
- Traini, T. et al. (2007). J Periodontol. 78(5): 955–61.(Case study)
- Nandi, SK. et al. (2010). Indian J Med Res. 132: 15–30.(Review)
- Kurien, T. et al. (2013). Bone Joint J. 95-B(5): 583–97. (Review)
How Orthoss® Works
References:
- Schlickewei, W. et al. Hefte zur Unfallkunde,216,(1991).(Case series)
- Bereiter, H. et al. (1991). Hefte zur Unfallkunde,216.(Expert opinion)
- Data on file at Geistlich Pharma AG, Wolhusen, Switzerland.(Bench Test)
- Kouroupis, D. et al. (2013). J Orthop Res. 31(12): 1950–8.(In Vitro)
- Orsini, G. et al. (2005). J Biomed Mater Res B Appl Biomater. 74(1): 448–7.(Case series)
- Traini, T. et al. (2007). J Periodontol. 78(5): 955–61.(Case study)
- Nandi, SK. et al. (2010). Indian J Med Res. 132: 15–30.(Review)
- Kurien, T. et al. (2013). Bone Joint J. 95-B(5): 583–97. (Review)
Bone substitute
To repair bone defects, human bone grafts are still widely considered the gold standard. But with both autografts and allografts, there are several known risks and disadvantages. These include the risk of disease transmission, donor site pain, and the limited availability or quality of material. 7,8
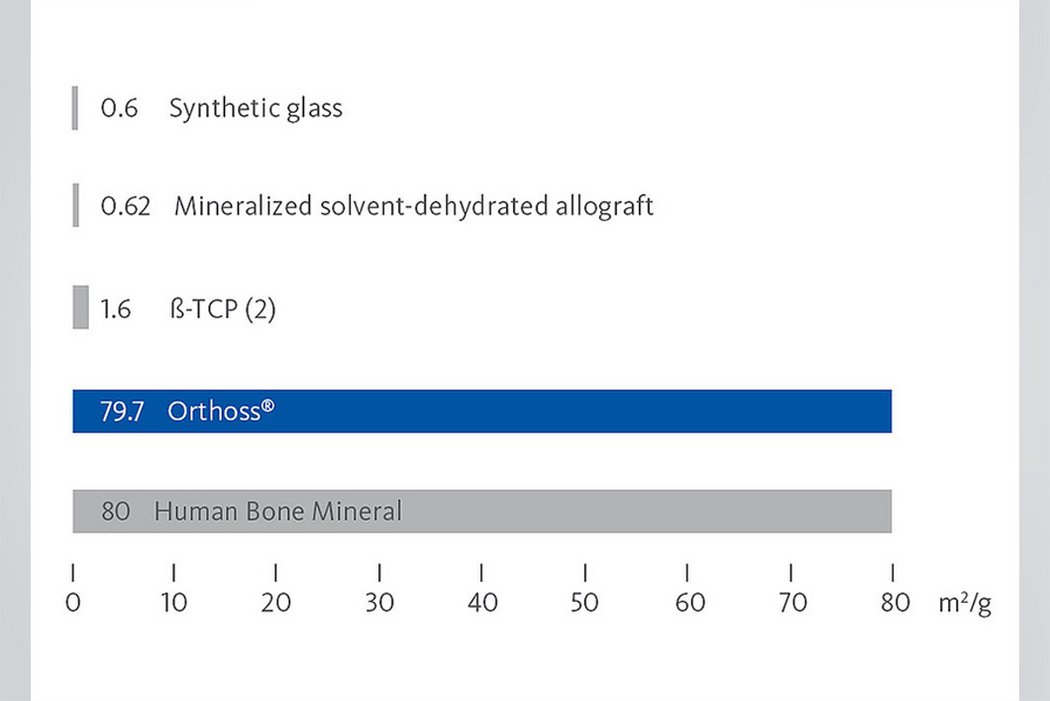
To ensure the quality and safety of a procedure, a bone substitute may be preferable. To see how Orthoss® compares with the other main types of bone substitutes currently used, view the graph below.3
The interconnected pore system and high porosity of Orthoss® results in an inner surface area that is significantly larger than other available bone graft substitutes and is similar to that of autologous bone mineral.
References:
- Schlickewei, W. et al. Hefte zur Unfallkunde,216,(1991).(Case series)
- Bereiter, H. et al. (1991). Hefte zur Unfallkunde,216.(Expert opinion)
- Data on file at Geistlich Pharma AG, Wolhusen, Switzerland.(Bench Test)
- Kouroupis, D. et al. (2013). J Orthop Res. 31(12): 1950–8.(In Vitro)
- Orsini, G. et al. (2005). J Biomed Mater Res B Appl Biomater. 74(1): 448–7.(Case series)
- Traini, T. et al. (2007). J Periodontol. 78(5): 955–61.(Case study)
- Nandi, SK. et al. (2010). Indian J Med Res. 132: 15–30.(Review)
- Kurien, T. et al. (2013). Bone Joint J. 95-B(5): 583–97. (Review)
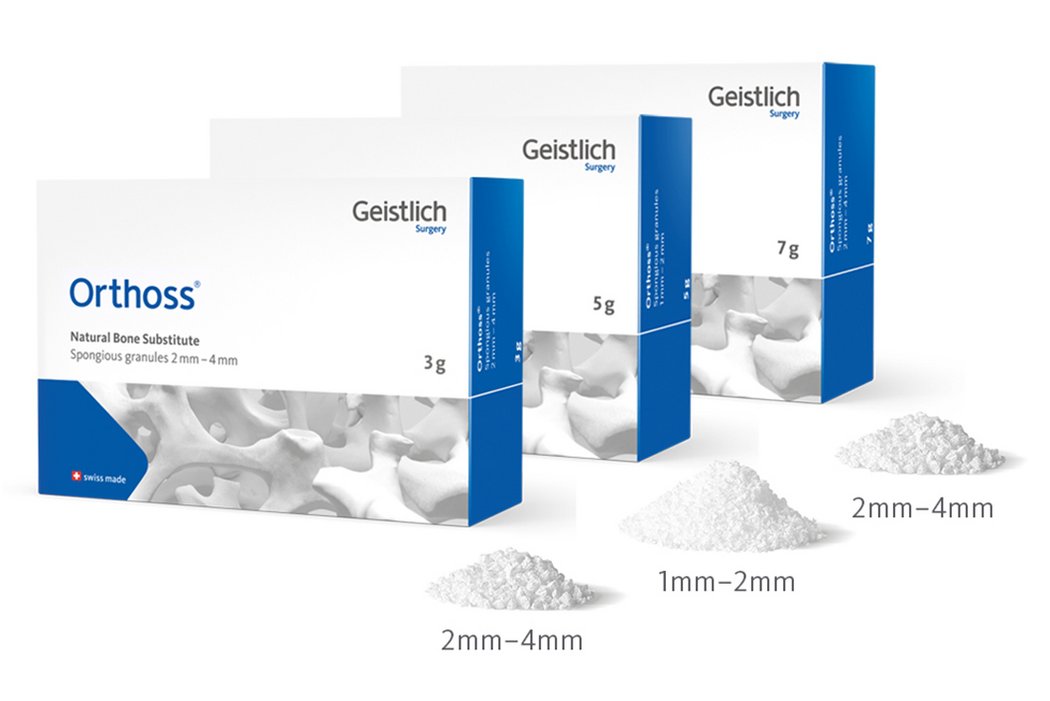
Available in Granules and Blocks