Allodyn® Allograft

User Benefits
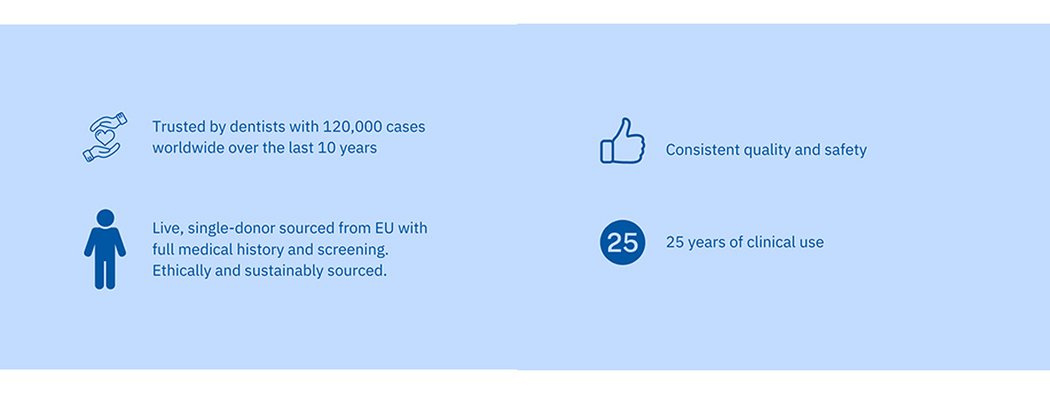
Allodyn® allograft delivers trusted bone regeneration
Leading clinicians have relied on Allodyn® allograft for over 25 years and within dentistry for over 10 years with over 120,000 cases worldwide. Every package of Allodyn® is sourced from a single, live, EU donor. Prior to donation, patients are medically certified as suitable to enter the donor programme, having undergone full lifestyle, travel and medical history check, each one undergoing further checks by using EU certified serology test equipment. Every package of Allodyn® allograft can be fully traced to each individual donor.
References
- Poumarat G, Thiery C, Toumi H, Abdi M, Garcier JM, Vanneuville G. Mechanical properties of human femoral head allografts after physico-chemical treatment (Osteopure). Rev Chir Orthop Reparatrice Appar Mot. 2004 Sep;90(5):442-8. French. doi: 10.1016/s0035-1040(04)70171-3. PMID: 15502767.
- Villatte et al. Orthopaedic & Traumatology: Surgery & Research 2015:101 953-957.
- OST Laboratories Data on file
- Chappard D et al, Fat in bone xenografts, Biomaterials, 1993:14(7):507-512
- Baslé MF et al, Shape and orientation of osteoblast-like cells (Saos-2) are influenced by collagen fibers in xenogenic bone biomaterial. J. Biomed. Mater.Res, 1998:40:350-357
- Moreau MF at el, Gamma irradiation of human bone allografts alters medullary lipids and releases toxic compounds for osteoblast-like cells, Biomaterials, 2000:21:369-376.
- Erivan et al. Rehydration improves the ductility of dry bone allografts. Cell Tissue Bank 2017:18, 307–312.
- Surmenian J et al, Three-Dimensional Reconstruction of the Posterior Mandible After Implant Removal: A Case Report of a Simplified Protocol. Clin Adv Periodontics.2018; 00:1-6.
- Dr Chamil Ardoin, Royan, France. 10. University of Bordeaux, Identification of allogenic and xeno/allogeneic grafts in dental biopsies, 2016.
Osteopure™ Process
The OSTEOPURE™ process consists of several scientifically designed steps; proven to provide you with the best quality of bone allografts.
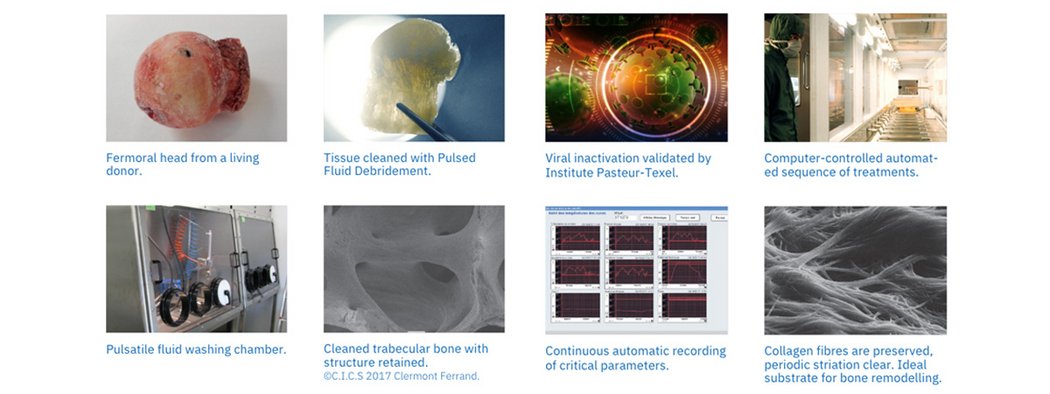
1. Pulsed Fluid Debridement
Highly trained technicians inspect each femoral head by hand and use pulsatile lavage to thoroughly clean and debride the tissue. Pulsatile lavage eliminates poor quality tissue while preserving healthy bone.
2. Viral Inactivation
Viral inactivation is achieved via a unique and patented high-tech, computer-controlled process, which ensures the safety of the product whilst preserving the structure.

3. Molecular Substitution Dehydration
A method that retains the structural integrity of the bone whilst removing water. Molecular Substitution replaces water in the tissue at room temperature by using ethanol. Freeze drying water from bone causes damage to the structure, whereas ethanol can be gently evaporated with the added benefit of acting as a disinfectant.
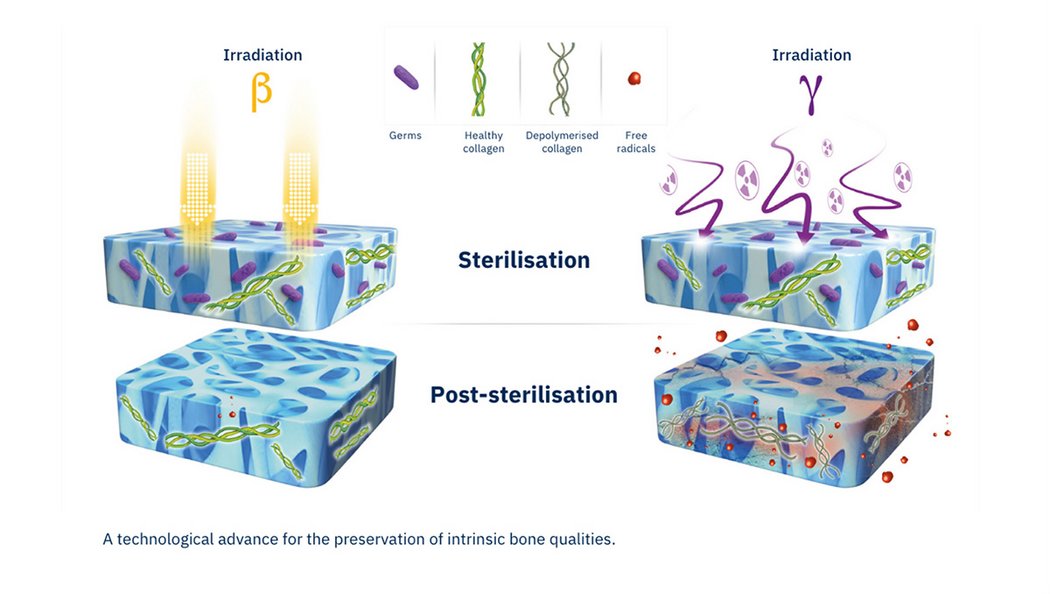
4. Electron Beam Sterilisation
A modern gold-standard for allograft sterilisation which ensures the highest level of sterility assurance. (Validation standard ISO 15011137 according to VDmax25 process, identical to gamma radiation, sterility assurance to industry standard level of 10-6, no exposure to a radioactive source (Cobalt 60, Cesium 137).
The short-duration exposure of the products means reduced heating and formation of free radicals within the allograft decreasing the depolymerisation of collagen from the bone.
Application
Simple and precise handling with Allodyn®
Allodyn® offers user-friendly application and reliable performance across a range of clinical indications.
- Prior to use, Allodyn® may be hydrated to enhance flexibility and ease of placement.
- The granules adapt to the defect morphology, ensuring optimal coverage and intimate contact with surrounding tissue.
- Gentle handling is recommended to maintain the integrity of the natural collagen structure and facilitate tissue integration.
- Allodyn® can be used in combination with a Geistlich barrier membrane such as Geistlich Bio-Gide® to support predictable soft-tissue healing and guided bone regeneration.
Product Range
Allodyn® offers a comprehensive range of allogeneic bone graft formats: granules, blocks, and plates: designed to suit various defect morphologies and clinical needs.
Granules
Allodyn® granules are available in various particle sizes:
| Granules | ||
|---|---|---|
| Allodyn® SP Fine | Cancellous granules (250–800µm) | 0.25, 0.5, 1.0cc |
| Allodyn® SP Std | Cancellous granules (800-1500µm) | 0.5, 1.0, 2.0cc |
| Allodyn® CS Fine | Cortico-cancellous granules (250–800µm) | 0.5, 1.0, 2.0cc |
| Allodyn® CT Fine | Cortical granules (250–800µm) | 0.5cc |
Blocks and Plates
For defects requiring structural support, Allodyn® includes block and plate formats:
| Blocks and Plates | ||
|---|---|---|
| Allodyn® Expand CS | Cortico-cancellous block | 20x10x5mm* |
| Allodyn® Expand CT | Cortical plate | 20x10x2mm* |
| Allodyn® Expand SP | Cancellous block | 25x12x10mm |
