Geistlich Select: REGENFAST®

Unlock your full regenerative potential with REGENFAST®
Whether periodontal, bone or soft-tissue regeneration, early healing is one of the most crucial aspects in ensuring the success of regenerative treatments. Patients generally want an approach that will lead to rapid healing and their treatment ending sooner rather than later. At the same time, predictability of clinical outcomes is decisive for long-term success.
Based on these needs, the influence of “regeneration boosters”, such as biologics and growth factors has grown considerably over the last years. Geistlich’s answer to this best practice shift is to introduce REGENFAST®, the first viscoelastic gel in dentistry featuring a unique combination of polynucleotides and hyaluronic acid.1
REGENFAST® is designed to protect the tissues of the oral cavity and to promote a rapid and physiological repair.1,2
References
- Mastelli: REGENFAST IFU 2023.
- Beretta M, et al.: Int. J. Periodontics Restor. Dent. 2024; 0(0): 1–23.
- Cairo F, et al.: Int. J. Periodontics Restor. Dent. 2024; 0(0): 1–24.
- Colangelo MT, et al.: Appl Sci 2021; 11(10): 4405.
- Colangelo MT, et al.: Appl Sci 2023; 13(2): 743.
- Vermeulen V: Data on File Geistlich Pharma AG 2023.
- Hämmerle CHF, et al.: Clinical oral implants research 2008; 19: 19–25.
- Meijndert L, et al.: International journal of oral and maxillofacial surgery 2005; 34(8): 877–84.
- Stagni C, et al.: BMC Musculoskelet. Disord. 2021; 22(1): 773.
- De Caridi G, et al.: Int. Wound J. 2016; 13(5): 754–58.
- Pilloni A, et al.: J Periodontol 2023; 94(3): 354–63.
- Colangelo MT, et al.: J Biol Reg Homeos Ag 2021; 35(1): 355–62.
- Vanelli R, et al.: Knee Surg Sports Traumatol Arthrosc 2010; 18: 901–07.
- Cavallini M, Papagni M: Journal of Plastic Dermatology 2007; 3(3): 27–32.
- Mathes SH, et al.: Biotechnol Bioeng 2010; 107(6): 1029–39.
- Thoma DS, et al.: Clin Oral Implants Res 2012; 23(12): 160–68.
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52.
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75.
References
- Mastelli: REGENFAST IFU 2023.
- Beretta M, et al.: Int. J. periodontics Restor. Dent. 2024; 0(0): 1–23.
- Cairo F, et al.: Int. J. Periodontics Restor. Dent. 2024; 0(0): 1–24.
- Colangelo MT, et al.: Appl Sci 2021; 11(10): 4405.
- Colangelo MT, et al.: Appl Sci 2023; 13(2): 743.
- Vermeulen V: Data on File Geistlich Pharma AG 2023.
- Hämmerle CHF, et al.: Clinical oral implants research 2008; 19: 19–25.
- Meijndert L, et al.: International journal of oral and maxillofacial surgery 2005; 34(8): 877–84.
- Stagni C, et al.: BMC Musculoskelet. Disord. 2021; 22(1): 773.
- De Caridi G, et al.: Int. Wound J. 2016; 13(5): 754–58.
- Pilloni A, et al.: J Periodontol 2023; 94(3): 354–63.
- Colangelo MT, et al.: J Biol Reg Homeos Ag 2021; 35(1): 355–62.
- Vanelli R, et al.: Knee Surg Sports Traumatol Arthrosc 2010; 18: 901–07.
- Cavallini M, Papagni M: Journal of Plastic Dermatology 2007; 3(3): 27–32.
- Mathes SH, et al.: Biotechnol Bioeng 2010; 107(6): 1029–39.
- Thoma DS, et al.: Clin Oral Implants Res 2012; 23(12): 160–68.
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52.
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75.
Discover the power of Bioregeneration
Starting in 2021, we have taken regeneration to a whole new level: Bioregeneration. But what exactly is it?
Bioregeneration is the enhancement and optimization of the physiological activities of cells involved in the natural regenerative processes of tissues in the human body.
How? Through the action of organic molecules known as polynucleotides.2
The polynucleotides (PN) contained in REGENFAST® are organic molecules of natural origin, highly purified and totally resorbable.3 Polynucleotides have several biological properties that make them the protagonists of bioregeneration:
References
- Mastelli: REGENFAST IFU 2023.
- Beretta M, et al.: Int. J. periodontics Restor. Dent. 2024; 0(0): 1–23.
- Cairo F, et al.: Int. J. Periodontics Restor. Dent. 2024; 0(0): 1–24.
- Colangelo MT, et al.: Appl Sci 2021; 11(10): 4405.
- Colangelo MT, et al.: Appl Sci 2023; 13(2): 743.
- Vermeulen V: Data on File Geistlich Pharma AG 2023.
- Hämmerle CHF, et al.: Clinical oral implants research 2008; 19: 19–25.
- Meijndert L, et al.: International journal of oral and maxillofacial surgery 2005; 34(8): 877–84.
- Stagni C, et al.: BMC Musculoskelet. Disord. 2021; 22(1): 773.
- De Caridi G, et al.: Int. Wound J. 2016; 13(5): 754–58.
- Pilloni A, et al.: J Periodontol 2023; 94(3): 354–63.
- Colangelo MT, et al.: J Biol Reg Homeos Ag 2021; 35(1): 355–62.
- Vanelli R, et al.: Knee Surg Sports Traumatol Arthrosc 2010; 18: 901–07.
- Cavallini M, Papagni M: Journal of Plastic Dermatology 2007; 3(3): 27–32.
- Mathes SH, et al.: Biotechnol Bioeng 2010; 107(6): 1029–39.
- Thoma DS, et al.: Clin Oral Implants Res 2012; 23(12): 160–68.
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52.
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75.
References
- Mastelli: REGENFAST IFU 2023.
- Beretta M, et al.: Int. J. periodontics Restor. Dent. 2024; 0(0): 1–23.
- Cairo F, et al.: Int. J. Periodontics Restor. Dent. 2024; 0(0): 1–24.
- Colangelo MT, et al.: Appl Sci 2021; 11(10): 4405.
- Colangelo MT, et al.: Appl Sci 2023; 13(2): 743.
- Vermeulen V: Data on File Geistlich Pharma AG 2023.
- Hämmerle CHF, et al.: Clinical oral implants research 2008; 19: 19–25.
- Meijndert L, et al.: International journal of oral and maxillofacial surgery 2005; 34(8): 877–84.
- Stagni C, et al.: BMC Musculoskelet. Disord. 2021; 22(1): 773.
- De Caridi G, et al.: Int. Wound J. 2016; 13(5): 754–58.
- Pilloni A, et al.: J Periodontol 2023; 94(3): 354–63.
- Colangelo MT, et al.: J Biol Reg Homeos Ag 2021; 35(1): 355–62.
- Vanelli R, et al.: Knee Surg Sports Traumatol Arthrosc 2010; 18: 901–07.
- Cavallini M, Papagni M: Journal of Plastic Dermatology 2007; 3(3): 27–32.
- Mathes SH, et al.: Biotechnol Bioeng 2010; 107(6): 1029–39.
- Thoma DS, et al.: Clin Oral Implants Res 2012; 23(12): 160–68.
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52.
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75.
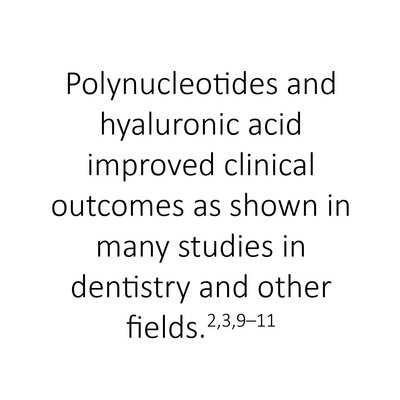
REGENFAST® unites polynucleotides with hyaluronic acid, using their synergistic effects to stimulate oral tissue repair and promote cell growth and vitality.2–5
References
- Mastelli: REGENFAST IFU 2023.
- Beretta M, et al.: Int. J. periodontics Restor. Dent. 2024; 0(0): 1–23.
- Cairo F, et al.: Int. J. Periodontics Restor. Dent. 2024; 0(0): 1–24.
- Colangelo MT, et al.: Appl Sci 2021; 11(10): 4405.
- Colangelo MT, et al.: Appl Sci 2023; 13(2): 743.
- Vermeulen V: Data on File Geistlich Pharma AG 2023.
- Hämmerle CHF, et al.: Clinical oral implants research 2008; 19: 19–25.
- Meijndert L, et al.: International journal of oral and maxillofacial surgery 2005; 34(8): 877–84.
- Stagni C, et al.: BMC Musculoskelet. Disord. 2021; 22(1): 773.
- De Caridi G, et al.: Int. Wound J. 2016; 13(5): 754–58.
- Pilloni A, et al.: J Periodontol 2023; 94(3): 354–63.
- Colangelo MT, et al.: J Biol Reg Homeos Ag 2021; 35(1): 355–62.
- Vanelli R, et al.: Knee Surg Sports Traumatol Arthrosc 2010; 18: 901–07.
- Cavallini M, Papagni M: Journal of Plastic Dermatology 2007; 3(3): 27–32.
- Mathes SH, et al.: Biotechnol Bioeng 2010; 107(6): 1029–39.
- Thoma DS, et al.: Clin Oral Implants Res 2012; 23(12): 160–68.
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52.
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75.
References
- Mastelli: REGENFAST IFU 2023.
- Beretta M, et al.: Int. J. periodontics Restor. Dent. 2024; 0(0): 1–23.
- Cairo F, et al.: Int. J. Periodontics Restor. Dent. 2024; 0(0): 1–24.
- Colangelo MT, et al.: Appl Sci 2021; 11(10): 4405.
- Colangelo MT, et al.: Appl Sci 2023; 13(2): 743.
- Vermeulen V: Data on File Geistlich Pharma AG 2023.
- Hämmerle CHF, et al.: Clinical oral implants research 2008; 19: 19–25.
- Meijndert L, et al.: International journal of oral and maxillofacial surgery 2005; 34(8): 877–84.
- Stagni C, et al.: BMC Musculoskelet. Disord. 2021; 22(1): 773.
- De Caridi G, et al.: Int. Wound J. 2016; 13(5): 754–58.
- Pilloni A, et al.: J Periodontol 2023; 94(3): 354–63.
- Colangelo MT, et al.: J Biol Reg Homeos Ag 2021; 35(1): 355–62.
- Vanelli R, et al.: Knee Surg Sports Traumatol Arthrosc 2010; 18: 901–07.
- Cavallini M, Papagni M: Journal of Plastic Dermatology 2007; 3(3): 27–32.
- Mathes SH, et al.: Biotechnol Bioeng 2010; 107(6): 1029–39.
- Thoma DS, et al.: Clin Oral Implants Res 2012; 23(12): 160–68.
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52.
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75.
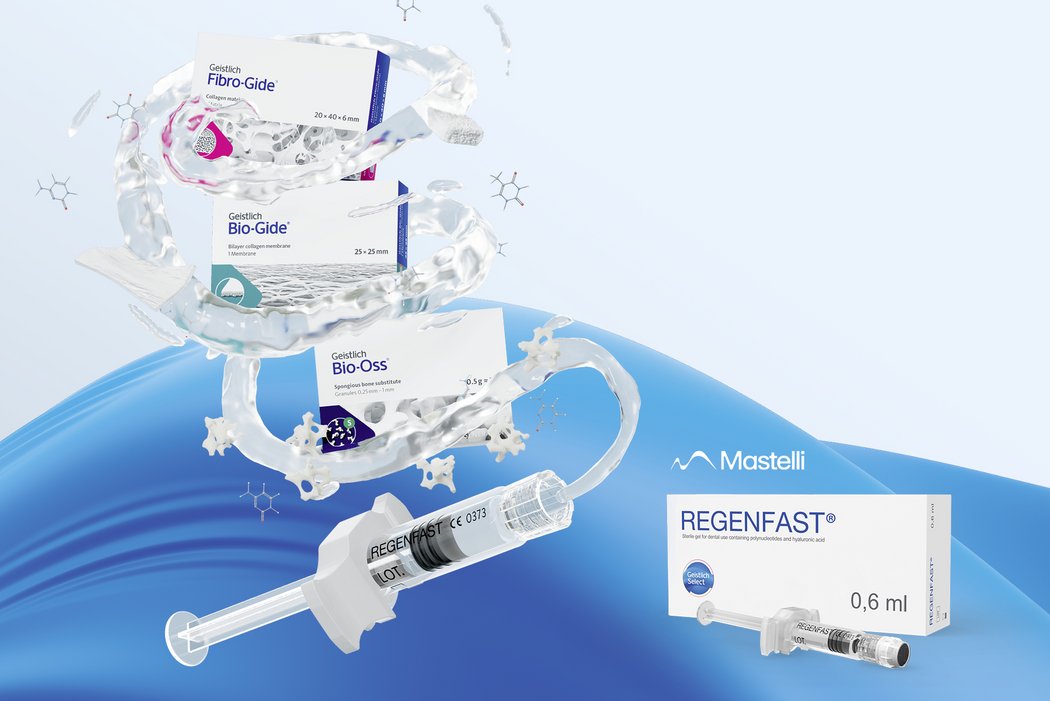
Part of a treatment solution
REGENFAST® has been used with Geistlich biomaterials in a wide range of clinical applications.6
References
- Mastelli: REGENFAST IFU 2023.
- Beretta M, et al.: Int. J. periodontics Restor. Dent. 2024; 0(0): 1–23.
- Cairo F, et al.: Int. J. Periodontics Restor. Dent. 2024; 0(0): 1–24.
- Colangelo MT, et al.: Appl Sci 2021; 11(10): 4405.
- Colangelo MT, et al.: Appl Sci 2023; 13(2): 743.
- Vermeulen V: Data on File Geistlich Pharma AG 2023.
- Hämmerle CHF, et al.: Clinical oral implants research 2008; 19: 19–25.
- Meijndert L, et al.: International journal of oral and maxillofacial surgery 2005; 34(8): 877–84.
- Stagni C, et al.: BMC Musculoskelet. Disord. 2021; 22(1): 773.
- De Caridi G, et al.: Int. Wound J. 2016; 13(5): 754–58.
- Pilloni A, et al.: J Periodontol 2023; 94(3): 354–63.
- Colangelo MT, et al.: J Biol Reg Homeos Ag 2021; 35(1): 355–62.
- Vanelli R, et al.: Knee Surg Sports Traumatol Arthrosc 2010; 18: 901–07.
- Cavallini M, Papagni M: Journal of Plastic Dermatology 2007; 3(3): 27–32.
- Mathes SH, et al.: Biotechnol Bioeng 2010; 107(6): 1029–39.
- Thoma DS, et al.: Clin Oral Implants Res 2012; 23(12): 160–68.
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52.
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75.

REGENFAST® decreased re-entry time in staged alveolar ridge preservation2
A recent study by Dr. Mario Beretta’s group in Italy has investigated the use of REGENFAST® to promote bone regeneration in horizontal alveolar defects.
The result: REGENFAST® shortened healing time in guided bone regeneration (GBR) treatments while supporting the formation of a high-quality regenerated bone2
The best practice waiting time before re-entry surgery in staged alveolar ridge augmentation procedures is 7-10 months.7 REGENFAST® used concurrently with Geistlich Bio-Oss® and Geistlich Bio-Gide® allowed stable implant placement after 5 months in this treatment protocol.2
The observed bone quality 5 months after treatment with REGENFAST® and Geistlich Bio-Oss® was higher than what would be expected after 6 months when using the bone substitute alone.2,8
References
- Mastelli: REGENFAST IFU 2023.
- Beretta M, et al.: Int. J. periodontics Restor. Dent. 2024; 0(0): 1–23.
- Cairo F, et al.: Int. J. Periodontics Restor. Dent. 2024; 0(0): 1–24.
- Colangelo MT, et al.: Appl Sci 2021; 11(10): 4405.
- Colangelo MT, et al.: Appl Sci 2023; 13(2): 743.
- Vermeulen V: Data on File Geistlich Pharma AG 2023.
- Hämmerle CHF, et al.: Clinical oral implants research 2008; 19: 19–25.
- Meijndert L, et al.: International journal of oral and maxillofacial surgery 2005; 34(8): 877–84.
- Stagni C, et al.: BMC Musculoskelet. Disord. 2021; 22(1): 773.
- De Caridi G, et al.: Int. Wound J. 2016; 13(5): 754–58.
- Pilloni A, et al.: J Periodontol 2023; 94(3): 354–63.
- Colangelo MT, et al.: J Biol Reg Homeos Ag 2021; 35(1): 355–62.
- Vanelli R, et al.: Knee Surg Sports Traumatol Arthrosc 2010; 18: 901–07.
- Cavallini M, Papagni M: Journal of Plastic Dermatology 2007; 3(3): 27–32.
- Mathes SH, et al.: Biotechnol Bioeng 2010; 107(6): 1029–39.
- Thoma DS, et al.: Clin Oral Implants Res 2012; 23(12): 160–68.
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52.
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75.
REGENFAST® is available in two different sizes
(Product availability may vary by country and region. Please contact your country representative of Geistlich Pharma AG for further information.)