Geistlich Bio-Oss®

The Most Trusted Bone Substitute For Dental Implants
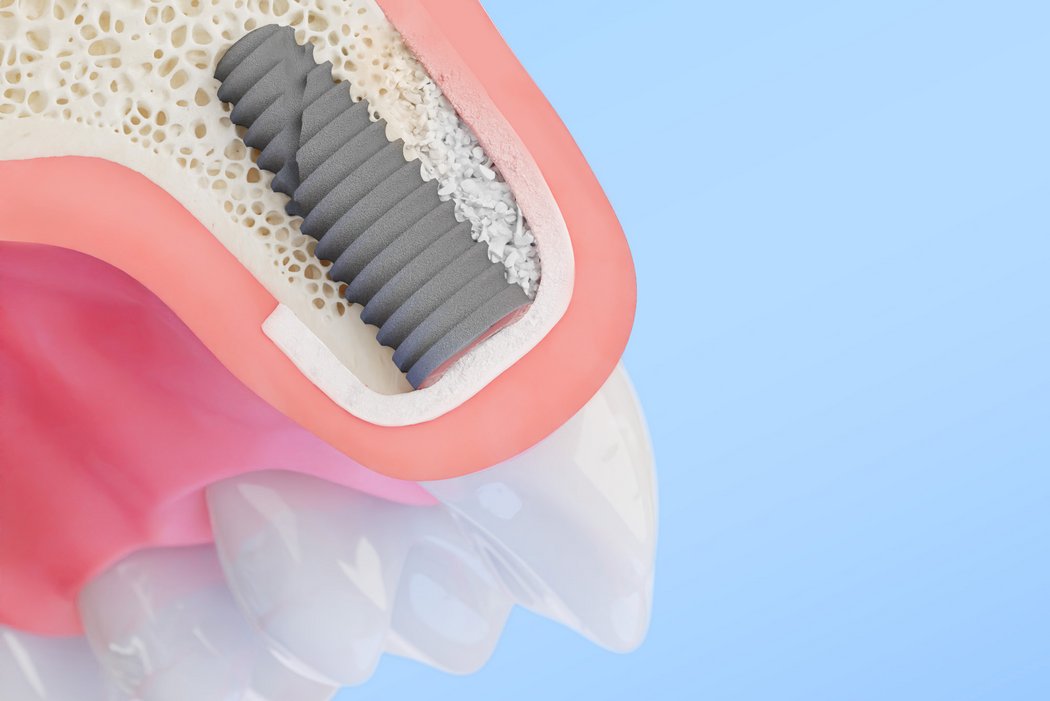
Higher implant survival and volume stability start with Geistlich Bio-Oss® – the original natural bone graft substitute for dental implants and bone regeneration. Its bovine-derived granules provide outstanding osteoconductivity, supporting predictable and effective bone regeneration for long-term clinical success. Recognized by clinicians worldwide, it is a reliable dental bone grafting material for a broad range of indications.1-11
References:
- iData Research, China market report suite for dental bone graft substitutes and other biomaterials, iDATA_CHDBGS18_MS, Nov 2018. (market research)
- iData Research, Europe market report suite for dental bone graft substitutes and other biomaterials, iDATA_EUDBGS19_MS, Jul 2019. (market research)
- iData Research, US market report suite for dental bone graft substitutes and other biomaterials, iDATA_USDBGS19_MS, Jan 2019. (market research)
- Aghaloo TL, Moy PK: Int J Oral Maxillofac Implants 2007; 22 (Suppl): 49–70. (systematic review)
- Jung RE, et al.: Clin Oral Implants Res 2013; 24(10): 1065–73. (clinical study)
- Orsini G, et al.: J Biomed Mater Res B Appl Biomater 2005; 74(1): 448–57. (clinical study)
- Traini T, et al.: J Periodontol 2007; 78(5): 955–61. (clinical study)
- Mordenfeld A, et al.: Clin Oral Implants Res 2010; 21(9): 961–70. (clinical study)
- Maiorana C, et al.: Open Dent J 2011; 5: 71–78. (clinical study)
- Galindo-Moreno P, et al.: Clin Implant Dent Relat Res 2013; 15(6): 858–66. (clinical study)
- Chappuis V, et al.: J Dent Res 2018; 97(3): 266–74. (clinical study)
- Knoefler W, et al.: Int J Implant Dent 2016; 2(1): 25. (clinical study)
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
- Jiang D, et al.: J Periodontol 1999; 70(8): 834–9. (preclinical study)
- Beretta M, et al.: Int J Periodontics Restorative Dent 2025; 45(2): 209–19. (clinical study)
- GEM 21S® Instructions for Use
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75. (clinical study)
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52. (preclinical study)
- Jung RE, et al.: Clin Oral Implants Res 2021; 32(12): 1455–65. (clinical study)
- PubMed search 08 August 2025; search term “bio-oss” (1,402 publications), https://pubmed.ncbi.nlm.nih.gov/?term=bio-oss
- Urban IA, et al.: Int J Periodontics Restorative Dent 2013; 33(3): 299–307. (clinical study)
- Cosyn J, et al.: J Clin Periodontol 2012; 39(10): 979–86. (clinical study)
- Camelo M, et al.: Int J Periodontics Restorative Dent 1998; 18(4): 321–31. (clinical study)
- Lundgren D, Slotte C: J Clin Periodontol 1999; 26(1): 56–62. (clinical study)
- Cardaropoli D, et al.: Int J Periodontics Restorative Dent 2014; 34(5): 631–7. (clinical study)
Exceptional Results You Can Rely On
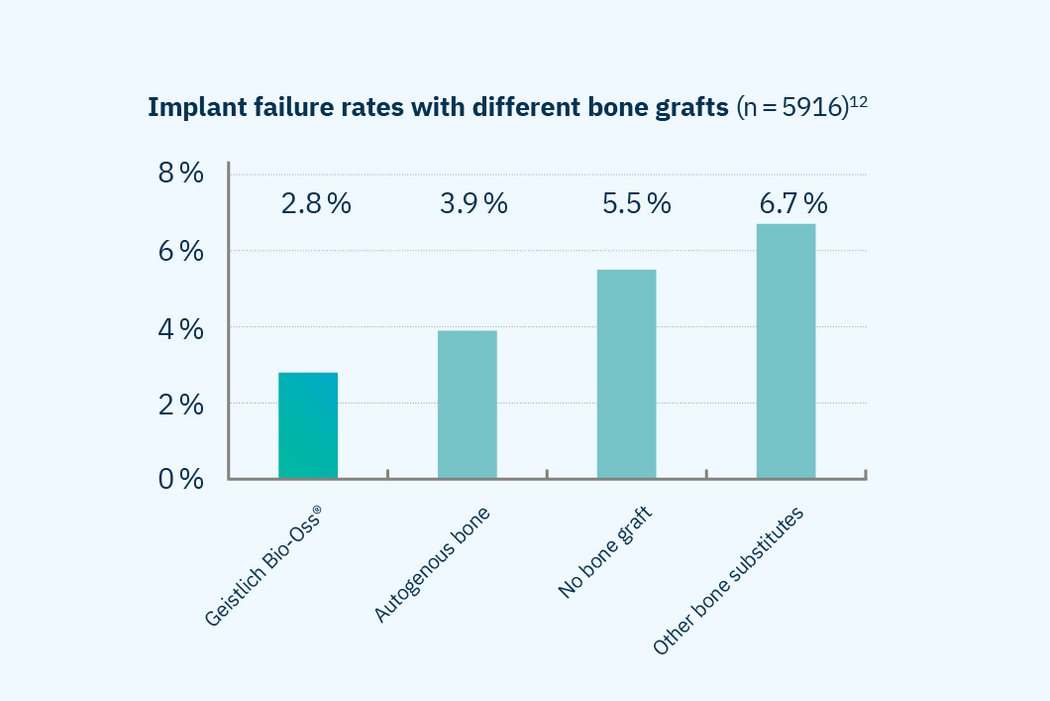
- Superior implant survival rate: Clinical evidence shows that using Geistlich Bio-Oss® as a bone graft material achieves implant survival rates above 97%, outperforming sites without grafting and those treated with other bone substitutes.12
- Unmatched volume stability: The slow resorption and high osteoconductivity of Geistlich Bio-Oss® support effective bone regeneration and long-term volume stability, forming a reliable base for excellent functional and esthetic outcomes.4-6,8,11
References:
- iData Research, China market report suite for dental bone graft substitutes and other biomaterials, iDATA_CHDBGS18_MS, Nov 2018. (market research)
- iData Research, Europe market report suite for dental bone graft substitutes and other biomaterials, iDATA_EUDBGS19_MS, Jul 2019. (market research)
- iData Research, US market report suite for dental bone graft substitutes and other biomaterials, iDATA_USDBGS19_MS, Jan 2019. (market research)
- Aghaloo TL, Moy PK: Int J Oral Maxillofac Implants 2007; 22 (Suppl): 49–70. (systematic review)
- Jung RE, et al.: Clin Oral Implants Res 2013; 24(10): 1065–73. (clinical study)
- Orsini G, et al.: J Biomed Mater Res B Appl Biomater 2005; 74(1): 448–57. (clinical study)
- Traini T, et al.: J Periodontol 2007; 78(5): 955–61. (clinical study)
- Mordenfeld A, et al.: Clin Oral Implants Res 2010; 21(9): 961–70. (clinical study)
- Maiorana C, et al.: Open Dent J 2011; 5: 71–78. (clinical study)
- Galindo-Moreno P, et al.: Clin Implant Dent Relat Res 2013; 15(6): 858–66. (clinical study)
- Chappuis V, et al.: J Dent Res 2018; 97(3): 266–74. (clinical study)
- Knoefler W, et al.: Int J Implant Dent 2016; 2(1): 25. (clinical study)
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
- Jiang D, et al.: J Periodontol 1999; 70(8): 834–9. (preclinical study)
- Beretta M, et al.: Int J Periodontics Restorative Dent 2025; 45(2): 209–19. (clinical study)
- GEM 21S® Instructions for Use
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75. (clinical study)
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52. (preclinical study)
- Jung RE, et al.: Clin Oral Implants Res 2021; 32(12): 1455–65. (clinical study)
- PubMed search 08 August 2025; search term “bio-oss” (1,402 publications), https://pubmed.ncbi.nlm.nih.gov/?term=bio-oss
- Urban IA, et al.: Int J Periodontics Restorative Dent 2013; 33(3): 299–307. (clinical study)
- Cosyn J, et al.: J Clin Periodontol 2012; 39(10): 979–86. (clinical study)
- Camelo M, et al.: Int J Periodontics Restorative Dent 1998; 18(4): 321–31. (clinical study)
- Lundgren D, Slotte C: J Clin Periodontol 1999; 26(1): 56–62. (clinical study)
- Cardaropoli D, et al.: Int J Periodontics Restorative Dent 2014; 34(5): 631–7. (clinical study)
Perform With User Friendly and Time-Saving Products
- Fast liquid uptake: Thanks to its unique micro- and macro-porous structure, Geistlich Bio-Oss® rapidly absorbs blood, nutrients, and growth factors—faster than other bone substitutes. The granules are hydrated within seconds, and their pores fill with serum proteins, serving as a natural growth factor reservoir.13,14
- Enhanced healing performance: The combination of Geistlich Bio-Oss® with autologous bone or biologics such as REGENFAST® or GEM 21S® promotes faster tissue regeneration.15,16 This effect is supported by the material’s osteoconductive properties, which make it an ideal scaffold for new bone formation.17,18
References:
- iData Research, China market report suite for dental bone graft substitutes and other biomaterials, iDATA_CHDBGS18_MS, Nov 2018. (market research)
- iData Research, Europe market report suite for dental bone graft substitutes and other biomaterials, iDATA_EUDBGS19_MS, Jul 2019. (market research)
- iData Research, US market report suite for dental bone graft substitutes and other biomaterials, iDATA_USDBGS19_MS, Jan 2019. (market research)
- Aghaloo TL, Moy PK: Int J Oral Maxillofac Implants 2007; 22 (Suppl): 49–70. (systematic review)
- Jung RE, et al.: Clin Oral Implants Res 2013; 24(10): 1065–73. (clinical study)
- Orsini G, et al.: J Biomed Mater Res B Appl Biomater 2005; 74(1): 448–57. (clinical study)
- Traini T, et al.: J Periodontol 2007; 78(5): 955–61. (clinical study)
- Mordenfeld A, et al.: Clin Oral Implants Res 2010; 21(9): 961–70. (clinical study)
- Maiorana C, et al.: Open Dent J 2011; 5: 71–78. (clinical study)
- Galindo-Moreno P, et al.: Clin Implant Dent Relat Res 2013; 15(6): 858–66. (clinical study)
- Chappuis V, et al.: J Dent Res 2018; 97(3): 266–74. (clinical study)
- Knoefler W, et al.: Int J Implant Dent 2016; 2(1): 25. (clinical study)
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
- Jiang D, et al.: J Periodontol 1999; 70(8): 834–9. (preclinical study)
- Beretta M, et al.: Int J Periodontics Restorative Dent 2025; 45(2): 209–19. (clinical study)
- GEM 21S® Instructions for Use
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75. (clinical study)
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52. (preclinical study)
- Jung RE, et al.: Clin Oral Implants Res 2021; 32(12): 1455–65. (clinical study)
- PubMed search 08 August 2025; search term “bio-oss” (1,402 publications), https://pubmed.ncbi.nlm.nih.gov/?term=bio-oss
- Urban IA, et al.: Int J Periodontics Restorative Dent 2013; 33(3): 299–307. (clinical study)
- Cosyn J, et al.: J Clin Periodontol 2012; 39(10): 979–86. (clinical study)
- Camelo M, et al.: Int J Periodontics Restorative Dent 1998; 18(4): 321–31. (clinical study)
- Lundgren D, Slotte C: J Clin Periodontol 1999; 26(1): 56–62. (clinical study)
- Cardaropoli D, et al.: Int J Periodontics Restorative Dent 2014; 34(5): 631–7. (clinical study)
Why Your Patients Will Love the Results
- Clinically proven and trusted: Geistlich Bio-Oss® is the most widely used and clinically documented dental bone graft substitute, with more than 21 million patients treated, and over two decades of documented success.1-3,13,19,20
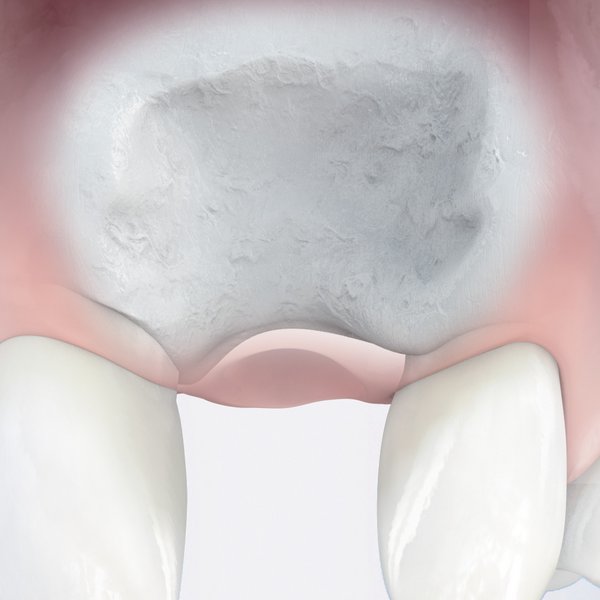
- Long-lasting esthetic results: Multiple studies confirm that Geistlich Bio-Oss® used with Geistlich Bio-Gide® leads to excellent long-term esthetic outcomes and stable peri‑implant health, even two decades after treatment.11,19
References:
- iData Research, China market report suite for dental bone graft substitutes and other biomaterials, iDATA_CHDBGS18_MS, Nov 2018. (market research)
- iData Research, Europe market report suite for dental bone graft substitutes and other biomaterials, iDATA_EUDBGS19_MS, Jul 2019. (market research)
- iData Research, US market report suite for dental bone graft substitutes and other biomaterials, iDATA_USDBGS19_MS, Jan 2019. (market research)
- Aghaloo TL, Moy PK: Int J Oral Maxillofac Implants 2007; 22 (Suppl): 49–70. (systematic review)
- Jung RE, et al.: Clin Oral Implants Res 2013; 24(10): 1065–73. (clinical study)
- Orsini G, et al.: J Biomed Mater Res B Appl Biomater 2005; 74(1): 448–57. (clinical study)
- Traini T, et al.: J Periodontol 2007; 78(5): 955–61. (clinical study)
- Mordenfeld A, et al.: Clin Oral Implants Res 2010; 21(9): 961–70. (clinical study)
- Maiorana C, et al.: Open Dent J 2011; 5: 71–78. (clinical study)
- Galindo-Moreno P, et al.: Clin Implant Dent Relat Res 2013; 15(6): 858–66. (clinical study)
- Chappuis V, et al.: J Dent Res 2018; 97(3): 266–74. (clinical study)
- Knoefler W, et al.: Int J Implant Dent 2016; 2(1): 25. (clinical study)
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
- Jiang D, et al.: J Periodontol 1999; 70(8): 834–9. (preclinical study)
- Beretta M, et al.: Int J Periodontics Restorative Dent 2025; 45(2): 209–19. (clinical study)
- GEM 21S® Instructions for Use
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75. (clinical study)
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52. (preclinical study)
- Jung RE, et al.: Clin Oral Implants Res 2021; 32(12): 1455–65. (clinical study)
- PubMed search 08 August 2025; search term “bio-oss” (1,402 publications), https://pubmed.ncbi.nlm.nih.gov/?term=bio-oss
- Urban IA, et al.: Int J Periodontics Restorative Dent 2013; 33(3): 299–307. (clinical study)
- Cosyn J, et al.: J Clin Periodontol 2012; 39(10): 979–86. (clinical study)
- Camelo M, et al.: Int J Periodontics Restorative Dent 1998; 18(4): 321–31. (clinical study)
- Lundgren D, Slotte C: J Clin Periodontol 1999; 26(1): 56–62. (clinical study)
- Cardaropoli D, et al.: Int J Periodontics Restorative Dent 2014; 34(5): 631–7. (clinical study)
Leading Dental Bone Graft Substitute Trusted Worldwide
For over 40 years, Geistlich Bio‑Oss® has helped clinicians maintain bone volume and implant stability with confidence.4-6,8,11,12 Its slow resorption supports predictable, long-term results where it matters most.4-6,8,11,12
References:
- iData Research, China market report suite for dental bone graft substitutes and other biomaterials, iDATA_CHDBGS18_MS, Nov 2018. (market research)
- iData Research, Europe market report suite for dental bone graft substitutes and other biomaterials, iDATA_EUDBGS19_MS, Jul 2019. (market research)
- iData Research, US market report suite for dental bone graft substitutes and other biomaterials, iDATA_USDBGS19_MS, Jan 2019. (market research)
- Aghaloo TL, Moy PK: Int J Oral Maxillofac Implants 2007; 22 (Suppl): 49–70. (systematic review)
- Jung RE, et al.: Clin Oral Implants Res 2013; 24(10): 1065–73. (clinical study)
- Orsini G, et al.: J Biomed Mater Res B Appl Biomater 2005; 74(1): 448–57. (clinical study)
- Traini T, et al.: J Periodontol 2007; 78(5): 955–61. (clinical study)
- Mordenfeld A, et al.: Clin Oral Implants Res 2010; 21(9): 961–70. (clinical study)
- Maiorana C, et al.: Open Dent J 2011; 5: 71–78. (clinical study)
- Galindo-Moreno P, et al.: Clin Implant Dent Relat Res 2013; 15(6): 858–66. (clinical study)
- Chappuis V, et al.: J Dent Res 2018; 97(3): 266–74. (clinical study)
- Knoefler W, et al.: Int J Implant Dent 2016; 2(1): 25. (clinical study)
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
- Jiang D, et al.: J Periodontol 1999; 70(8): 834–9. (preclinical study)
- Beretta M, et al.: Int J Periodontics Restorative Dent 2025; 45(2): 209–19. (clinical study)
- GEM 21S® Instructions for Use
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75. (clinical study)
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52. (preclinical study)
- Jung RE, et al.: Clin Oral Implants Res 2021; 32(12): 1455–65. (clinical study)
- PubMed search 08 August 2025; search term “bio-oss” (1,402 publications), https://pubmed.ncbi.nlm.nih.gov/?term=bio-oss
- Urban IA, et al.: Int J Periodontics Restorative Dent 2013; 33(3): 299–307. (clinical study)
- Cosyn J, et al.: J Clin Periodontol 2012; 39(10): 979–86. (clinical study)
- Camelo M, et al.: Int J Periodontics Restorative Dent 1998; 18(4): 321–31. (clinical study)
- Lundgren D, Slotte C: J Clin Periodontol 1999; 26(1): 56–62. (clinical study)
- Cardaropoli D, et al.: Int J Periodontics Restorative Dent 2014; 34(5): 631–7. (clinical study)
Geistlich Bio-Oss®
The original natural bone substitute
Fast Liquid Uptake
Geistlich Bio-Oss® granules can be hydrated with saline or blood.
Fast Liquid Uptake
The micro - and macropores of Geistlich Bio-Oss® enable a faster absorption of liquids, nutrients, and growth factors compared to other bone substitutes. Thanks to its high hydrophilicity, the granules become fully hydrated within seconds.
Boosting Performance
Combine the granules with autologous bone and/or biologics to promote a faster healing process.
The Dream Team
Multiple studies confirm that Geistlich Bio-Oss® and Geistlich Bio-Gide® deliver excellent long-term esthetic outcomes and peri-implant health – even 20+ years after the procedure.
Porous with a Purpose
One gram of Geistlich Bio-Oss® has a surface area of 80 square meters. Its highly porous structure rapidly absorbs and retains blood, serving as a reservoir for growth factors.
Unmatched Volume Stability
The outstanding osteoconductive properties and slow resorption rate of Geistlich Bio-Oss® promote effective bone regeneration and long-term volume stability.
Excellent Long-Term Implant Survival
Implant survival rates exceed 97% when using Geistlich Bio-Oss®, outperforming sites treated without graft material, with other bone substitutes, or with autologous bone.
Excellent Long-Term Implant Survival
>97% implant survival rate
Excellent Long-Term Implant Survival
Even after 20+ years
Your Advantages at a Glance
- Fast liquid uptake
- High osteoconductivity
- Unmatched volume stability
- Excellent implant survival rate
- Long-lasting esthetic results
Geistlich Bio-Oss® Collagen
You Can’t Cut Corners When Leading Regenerative Dentistry
Our Swiss-made products—like Geistlich Bio-Oss® —meet the highest quality standards in sourcing, production, and logistics and have undergone rigorous testing in over 2,300 studies worldwide to ensure their safety and efficacy.
Geistlich offers a comprehensive suite of resources for dental professionals. It provides a variety of training and courses, available in multiple formats, covering a wide range of topics, from regeneration biology to effective patient communication. Geistlich also offers patient education materials to help dentists explain treatment options and support informed decision-making.
Leading Experts Trust Geistlich Bio-Oss®
References
- iData Research, China market report suite for dental bone graft substitutes and other biomaterials, iDATA_CHDBGS18_MS, Nov 2018. (market research)
- iData Research, Europe market report suite for dental bone graft substitutes and other biomaterials, iDATA_EUDBGS19_MS, Jul 2019. (market research)
- iData Research, US market report suite for dental bone graft substitutes and other biomaterials, iDATA_USDBGS19_MS, Jan 2019. (market research)
- Aghaloo TL, Moy PK: Int J Oral Maxillofac Implants 2007; 22 (Suppl): 49–70. (systematic review)
- Jung RE, et al.: Clin Oral Implants Res 2013; 24(10): 1065–73. (clinical study)
- Orsini G, et al.: J Biomed Mater Res B Appl Biomater 2005; 74(1): 448–57. (clinical study)
- Traini T, et al.: J Periodontol 2007; 78(5): 955–61. (clinical study)
- Mordenfeld A, et al.: Clin Oral Implants Res 2010; 21(9): 961–70. (clinical study)
- Maiorana C, et al.: Open Dent J 2011; 5: 71–78. (clinical study)
- Galindo-Moreno P, et al.: Clin Implant Dent Relat Res 2013; 15(6): 858–66. (clinical study)
- Chappuis V, et al.: J Dent Res 2018; 97(3): 266–74. (clinical study)
- Knoefler W, et al.: Int J Implant Dent 2016; 2(1): 25. (clinical study)
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
- Jiang D, et al.: J Periodontol 1999; 70(8): 834–9. (preclinical study)
- Beretta M, et al.: Int J Periodontics Restorative Dent 2025; 45(2): 209–19. (clinical study)
- GEM 21S® Instructions for Use
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75. (clinical study)
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52. (preclinical study)
- Jung RE, et al.: Clin Oral Implants Res 2021; 32(12): 1455–65. (clinical study)
- PubMed search 08 August 2025; search term “bio-oss” (1,402 publications), https://pubmed.ncbi.nlm.nih.gov/?term=bio-oss
- Urban IA, et al.: Int J Periodontics Restorative Dent 2013; 33(3): 299–307. (clinical study)
- Cosyn J, et al.: J Clin Periodontol 2012; 39(10): 979–86. (clinical study)
- Camelo M, et al.: Int J Periodontics Restorative Dent 1998; 18(4): 321–31. (clinical study)
- Lundgren D, Slotte C: J Clin Periodontol 1999; 26(1): 56–62. (clinical study)
- Cardaropoli D, et al.: Int J Periodontics Restorative Dent 2014; 34(5): 631–7. (clinical study)
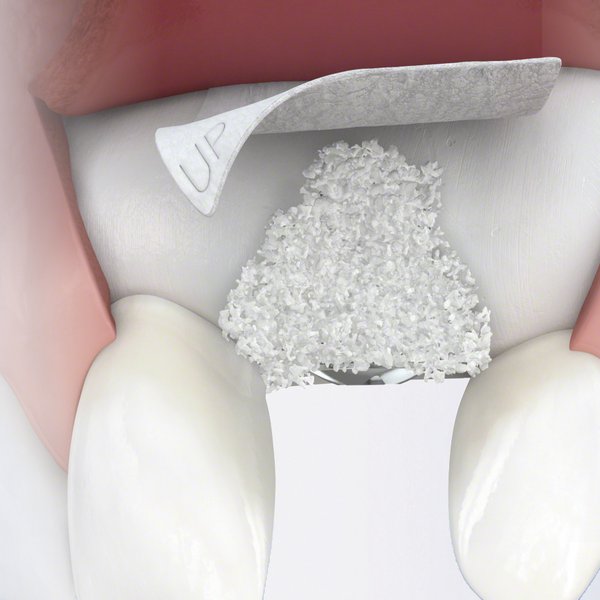
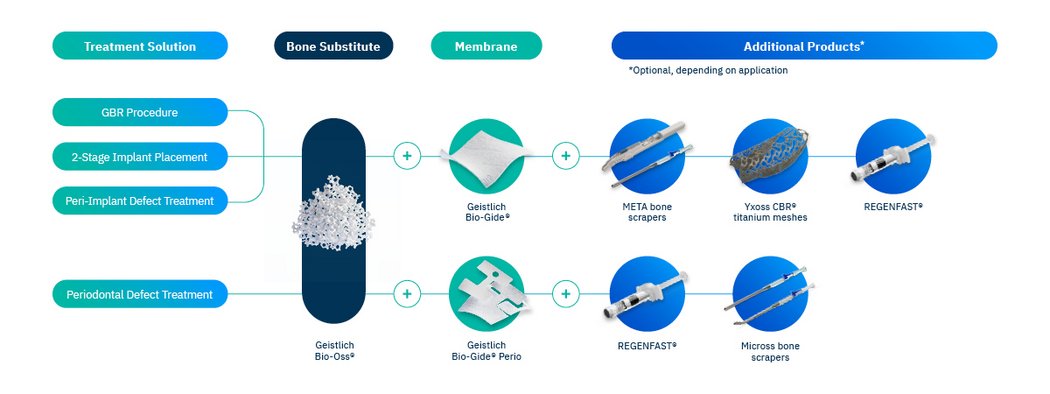
Versatile bone graft substitute for GBR, 2-stage implant placement, periodontal and peri-implant defect treatment
Geistlich Bio‑Oss® is a versatile dental bone grafting material suitable for Guided Bone Regeneration (GBR)11, 2‑stage implant placement21, and the treatment of periodontal22-24 and peri‑implant25 defects. Its adaptability and proven clinical performance make it a dependable choice across indications.11,21-25
References
- iData Research, China market report suite for dental bone graft substitutes and other biomaterials, iDATA_CHDBGS18_MS, Nov 2018. (market research)
- iData Research, Europe market report suite for dental bone graft substitutes and other biomaterials, iDATA_EUDBGS19_MS, Jul 2019. (market research)
- iData Research, US market report suite for dental bone graft substitutes and other biomaterials, iDATA_USDBGS19_MS, Jan 2019. (market research)
- Aghaloo TL, Moy PK: Int J Oral Maxillofac Implants 2007; 22 (Suppl): 49–70. (systematic review)
- Jung RE, et al.: Clin Oral Implants Res 2013; 24(10): 1065–73. (clinical study)
- Orsini G, et al.: J Biomed Mater Res B Appl Biomater 2005; 74(1): 448–57. (clinical study)
- Traini T, et al.: J Periodontol 2007; 78(5): 955–61. (clinical study)
- Mordenfeld A, et al.: Clin Oral Implants Res 2010; 21(9): 961–70. (clinical study)
- Maiorana C, et al.: Open Dent J 2011; 5: 71–78. (clinical study)
- Galindo-Moreno P, et al.: Clin Implant Dent Relat Res 2013; 15(6): 858–66. (clinical study)
- Chappuis V, et al.: J Dent Res 2018; 97(3): 266–74. (clinical study)
- Knoefler W, et al.: Int J Implant Dent 2016; 2(1): 25. (clinical study)
- Data on file. Geistlich Pharma AG, Wolhusen, Switzerland.
- Jiang D, et al.: J Periodontol 1999; 70(8): 834–9. (preclinical study)
- Beretta M, et al.: Int J Periodontics Restorative Dent 2025; 45(2): 209–19. (clinical study)
- GEM 21S® Instructions for Use
- Degidi M, et al.: Oral Dis 2006; 12(5): 469–75. (clinical study)
- Weibrich G, et al.: Mund Kiefer Gesichtschir 2000; 4(3): 148–52. (preclinical study)
- Jung RE, et al.: Clin Oral Implants Res 2021; 32(12): 1455–65. (clinical study)
- PubMed search 08 August 2025; search term “bio-oss” (1,402 publications), https://pubmed.ncbi.nlm.nih.gov/?term=bio-oss
- Urban IA, et al.: Int J Periodontics Restorative Dent 2013; 33(3): 299–307. (clinical study)
- Cosyn J, et al.: J Clin Periodontol 2012; 39(10): 979–86. (clinical study)
- Camelo M, et al.: Int J Periodontics Restorative Dent 1998; 18(4): 321–31. (clinical study)
- Lundgren D, Slotte C: J Clin Periodontol 1999; 26(1): 56–62. (clinical study)
- Cardaropoli D, et al.: Int J Periodontics Restorative Dent 2014; 34(5): 631–7. (clinical study)
Geistlich Bio-Oss®: Sizes and Granule Options
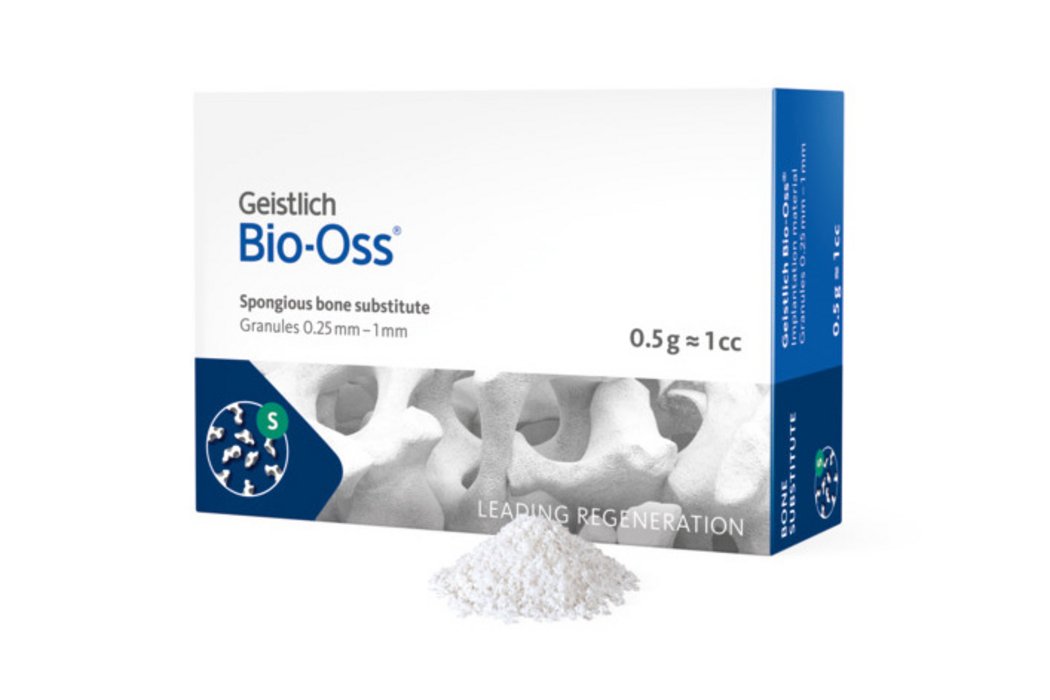
Geistlich Bio-Oss® is available in seven quantities with small or large granules to meet your clinical needs:
Small granules (0.25 – 1 mm): Adapt well to surface contours; especially suitable for smaller defects and for contouring around autogenous blocks. Available volumes: 0.25 g ≈ 0.5 cc; 0.5 g ≈ 1 cc; 1 g ≈ 2 cc; 2 g ≈ 4 cc.
Large granules (1 – 2 mm): Recommended for larger augmentations. Available volumes: 0.5 g ≈ 1.5 cc; 1 g ≈ 3 cc; 2 g ≈ 6 cc.
*Product availability may vary by country.